Current research activities in the lab are mainly focused on identifying critical mechanisms for musculoskeletal degeneration and regeneration, e.g., bone remodeling, and integrated basic sciences and applied translational research approaches for diagnoses and treatment of such skele-tal diseases, e.g., osteopenia, osteoporosis, and muscle atrophy. Current external funded research areas in the lab include: (1) identifying the osteogenic (bone producing) components induced by biophysical stimuli (e.g., mechanotransduction factors), and their potential for application in the treatment of skeletal disorders; and (2) using physical signals (e.g., ultrasound) as the basis for a non-invasive diagnostic the assessment of bone quality and quantity (i.e., Scanning Confocal Acoustic Navigation, or SCAN, technology). These research programs are currently funded by several federal agencies, including the National Institute of Health (2 R01 projects), the National Space Biomedical Research Institute (NSBRI) and The US Army Medical Research and Materiel Command (USAMRAA). Total of approximately $1M annual funding is currently facilitated to these research activities in the lab.
The motivation for these research projects are stemmed from the significant impact of skeletal diseases in our society. For example, osteoporosis has become a major health problem, and is the leading cause of bone fractures in elderly populations for both men and women. In the US alone, well over 25% of women aged 50 years and older, and over 5% percent of men aged 50 and over, suffer from symptomatic osteoporosis. In total, over 24 million people have osteoporosis, with an estimated annual direct cost approaching $20 billion to our national health programs. The skeleton is exquisitely sensitive to mechanical information and an improved understanding of how tissue quantity and quality is regulated and can be perturbed by mechanical signals may provide an unique opportunity to develop effective countermeasures of bone loss.
The motivation for these research projects are stemmed from the significant impact of skeletal diseases in our society. For example, osteoporosis has become a major health problem, and is the leading cause of bone fractures in elderly populations for both men and women. In the US alone, well over 25% of women aged 50 years and older, and over 5% percent of men aged 50 and over, suffer from symptomatic osteoporosis. In total, over 24 million people have osteoporosis, with an estimated annual direct cost approaching $20 billion to our national health programs. The skeleton is exquisitely sensitive to mechanical information and an improved understanding of how tissue quantity and quality is regulated and can be perturbed by mechanical signals may provide an unique opportunity to develop effective countermeasures of bone loss.
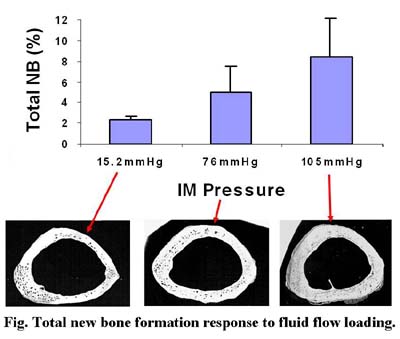
The ability of bone to respond to changes in its functional milieu is one of the most intriguing aspects of bioengineering, and certainly contributes to the skeleton's success as a structure. Bone's ability to rapidly accommodate changes in its functional environment ensures that sufficient skeletal mass is appropriately placed to withstand the rigors of functional activity, an attribute described as Wolff's Law. This adaptive capability of bone suggests that biophysical stimuli may be able to provide a site-specific, exogenous treatment for controlling both bone's mass and morphology. Our laboratory focuses on a strategy for the prevention and/or treatment of such skeletal complications based on harnessing bone tissue's sensitivity to its physical environment. More specifically, intracortical fluid flow, should it prove a key mediator of the osteogenic response, may open up unique interventional approach for the treatment of musculoskeletal disorders.
The responses to temporal components of mechanical stimuli on the skeleton are particularly interesting, given their relation to mechanocoupling between matrix and strain-generated fluid flow within bone. For instance, in the solid phase, strain gradients induce fluid pressure gradients within bone, which, in turn, generate fluid flow in the tissue. Fluid-induced flow movements have been proposed as a mechanism by which bone perceives mechanical signals in the mineralized tissue.
In addition to fluid flow's mechanical function in bone, it serves as a necessary pathway for metabolism in the tissue by providing an adequate supply of nutrients (and proper disposal of waste products). In soft tissue, molecular diffusion is considered the major pathway for transportation of metabolites. However, because of the relatively dense structure of cortical bone, a diffusive mechanism may not be sufficient to play an adequate role in transporting metabolic fluid between osteocytes and the surrounding vascular canals. Mechanical loading induced convectional fluid flow may enhance the transportation from the blood supply to osteocytes through this connective mechanism. These projects are currently funded by the US Army, and NIH.
The responses to temporal components of mechanical stimuli on the skeleton are particularly interesting, given their relation to mechanocoupling between matrix and strain-generated fluid flow within bone. For instance, in the solid phase, strain gradients induce fluid pressure gradients within bone, which, in turn, generate fluid flow in the tissue. Fluid-induced flow movements have been proposed as a mechanism by which bone perceives mechanical signals in the mineralized tissue.
In addition to fluid flow's mechanical function in bone, it serves as a necessary pathway for metabolism in the tissue by providing an adequate supply of nutrients (and proper disposal of waste products). In soft tissue, molecular diffusion is considered the major pathway for transportation of metabolites. However, because of the relatively dense structure of cortical bone, a diffusive mechanism may not be sufficient to play an adequate role in transporting metabolic fluid between osteocytes and the surrounding vascular canals. Mechanical loading induced convectional fluid flow may enhance the transportation from the blood supply to osteocytes through this connective mechanism. These projects are currently funded by the US Army, and NIH.
Development of Non-Invasive Scanning Confocal Acoustic Navigation (SCAN) System for the Determination of Bone Quantity and Quality
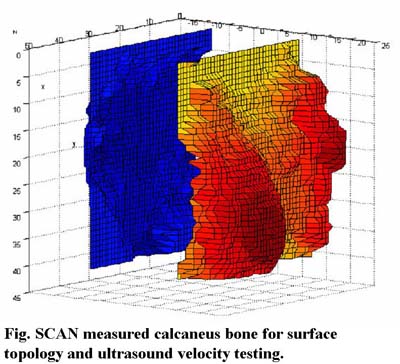
Due to technological and societal advancements, life expectancy has increased dramatically over the past 100 years, and today is nearly 79 years for women and 73 years for men (U.S. Department of Commerce, Bureau of Census). It is projected that life expectancy will increase by 5~7 years in the next 50 years, leading to an increased public health challenge to improve the quality of life (U.S. Department of Health and Human Services: Healthy People 2010). Chronic diseases, and in particular musculoskeletal complications, have a long-term debilitating effect that greatly impacts quality of life.
Osteoporosis is a reduction in bone mass and strength that leads to fragile bones. One-third of women over 65 will have vertebral fractures and 90% of women aged 75 and older have radiographic evidence of osteoporosis. Early detection, however, will allow early treatment. At present, osteoporosis is commonly assessed by measuring bone's quantity using bone mineral density (BMD), which provides a two-dimensional apparent density of a given bone structure. Noninvasive measurements of bone mass would be valuable in predicting the risk of fracture, in assessing the severity of the disease, and in following the response to treatment. Several methods are available for the measurement of bone mass, with the most common used methods being DXA and computerized tomography (CT).
DXA is the most commonly used technique because of its relative precision (~2%), and multi-access (spine, hip, wrist and total skeleton). However, because the source and detector cross whole bones (including layers of cortex and trabeculae), these techniques are insensitive to subtle changes in bone mass or architecture. Ultrasonic techniques (UT) provide an intriguing method for characterizing the physical properties of bone, in true three dimensions, since it is non-invasive, non-destructive, and relatively accurate. Quantitative ultrasound (QUS) is an emerging physical modality in the evaluation of bone material properties because it is simple, inexpensive, non-invasive and free of ionizing radiation. Preliminary results for predicting osteoporosis using QUS are promising, and it has great potential for widespread application.
Our research has led to a new design of ultrasound diagnostic, and this SCAN system is intended to provide true images reflecting bone's structural and strength properties in multi-skeletal sites, i.e., in hip, which can provide a true diagnostic tool (instead of screening) that will surpass the radiation based DXA machines. By using confocal ultrasonic beam at an appropriate frequency, the SCAN system allows for a significant improvement in UT resolution and sensitivity. Micro-scanning and imaging further improve the resolution in a given region of interest, to avoid any artifacts caused by the complex porous structure. Data has shown significant correlations between CT determined bone density and architecture parameters and SCAN measured bone quality. Current work includes the development of a rapid SCAN system, with the capability to identify bone surface topology for UV images and provide real-time predictions of bone strength and structural properties at multi skeletal sites, particularly those at risk of fracture, i.e., hip region. These projects are currently funded by the National Space Biomedical Research Institute, and the Coulter Foundation. With the goal of bringing the SCAN technology from the lab to bedside, AcousticScan, Inc., a new faculty based company, was founded in 2001. There is a patent pending and two provisional patents for this technology.
Osteoporosis is a reduction in bone mass and strength that leads to fragile bones. One-third of women over 65 will have vertebral fractures and 90% of women aged 75 and older have radiographic evidence of osteoporosis. Early detection, however, will allow early treatment. At present, osteoporosis is commonly assessed by measuring bone's quantity using bone mineral density (BMD), which provides a two-dimensional apparent density of a given bone structure. Noninvasive measurements of bone mass would be valuable in predicting the risk of fracture, in assessing the severity of the disease, and in following the response to treatment. Several methods are available for the measurement of bone mass, with the most common used methods being DXA and computerized tomography (CT).
DXA is the most commonly used technique because of its relative precision (~2%), and multi-access (spine, hip, wrist and total skeleton). However, because the source and detector cross whole bones (including layers of cortex and trabeculae), these techniques are insensitive to subtle changes in bone mass or architecture. Ultrasonic techniques (UT) provide an intriguing method for characterizing the physical properties of bone, in true three dimensions, since it is non-invasive, non-destructive, and relatively accurate. Quantitative ultrasound (QUS) is an emerging physical modality in the evaluation of bone material properties because it is simple, inexpensive, non-invasive and free of ionizing radiation. Preliminary results for predicting osteoporosis using QUS are promising, and it has great potential for widespread application.
Our research has led to a new design of ultrasound diagnostic, and this SCAN system is intended to provide true images reflecting bone's structural and strength properties in multi-skeletal sites, i.e., in hip, which can provide a true diagnostic tool (instead of screening) that will surpass the radiation based DXA machines. By using confocal ultrasonic beam at an appropriate frequency, the SCAN system allows for a significant improvement in UT resolution and sensitivity. Micro-scanning and imaging further improve the resolution in a given region of interest, to avoid any artifacts caused by the complex porous structure. Data has shown significant correlations between CT determined bone density and architecture parameters and SCAN measured bone quality. Current work includes the development of a rapid SCAN system, with the capability to identify bone surface topology for UV images and provide real-time predictions of bone strength and structural properties at multi skeletal sites, particularly those at risk of fracture, i.e., hip region. These projects are currently funded by the National Space Biomedical Research Institute, and the Coulter Foundation. With the goal of bringing the SCAN technology from the lab to bedside, AcousticScan, Inc., a new faculty based company, was founded in 2001. There is a patent pending and two provisional patents for this technology.
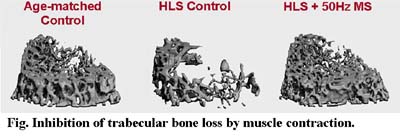
Using oscillatory pressurized marrow fluid flow stimuli, the physiological fluid stimulus was found to initiate new bone formation and reduce intracortical bone porosities caused by disuse, even in the absence of direct tissue strain. While bone remodeling was demonstrated to be sensitive to high rate of dynamic physiological stimulation, the role of fluid flow in both bone and muscle perhaps explains, at least in part, the cellular response mechanism to anabolic stimuli. Muscle stimulation via contractions has been shown to promote blood flow to bone, possibly by creating a pressure gradient within the microvasculature. Physiological fluid flow has the potential to mediate bone remodeling, initiate new bone formation and reduce intracortical porosities due to disuse condition. Elucidation of the interactive mechanism between musculoskeletal circulations and bone remodeling is crucial in developing new intervention to prevent bone loss under microgravity environment.
The overall hypothesis is that dynamic muscle stimulation can enhance fluid circulation in bone, regulate osteogenic adaptation, and inhibit bone loss in a functional disuse condition. Using a hindlimb suspension rat model, electro-induced dynamic muscle contraction was applied as replacement of the normal weight-bearing activity of the hindlimb. Dynamic muscle stimulation was induced with two needle-size electrodes at the right quadriceps muscles. The stimulus was applied at 1V, 1ms square pulse with a frequency of either 20Hz, 50Hz, or 100Hz, daily for 10minutes, 5days per week, for a total of 4weeks. Left and right femurs were harvested for micro-computed tomography analysis. Three distal metaphyseal regions and one epiphyseal region of the femurs (0.75mm per region) were scanned at 15 m resolution and evaluated to obtain bone volume fraction (BV/TV), connectivity (Conn.D), trabecular number (Tb.N). Dynamic muscle contraction loading at 50Hz demonstrated anabolic effects at the metaphyseal regions (+13% BV/TV, +32% Conn.D, +8% Tb.N, when comparing to the average sham control). Frequency at 20Hz showed a lesser response (+11% BV/TV, no change on Conn.D, +2% Tb.N). However, contraction at frequency of 100Hz generated adverse effects on the HLS skeleton. These results demonstrated that dynamic electro-induced muscle contraction can in deed initiate adaptive response to inhibit bone loss under functional disuse environment. The responses are depended on the applied loading frequency, suggesting that low-level bone strain together with increased intramedullary pressure may be crucial factors in understanding the interrelationship between dynamic muscle contraction and musculoskeletal tissue remodeling. This project is currently funded by NIH.
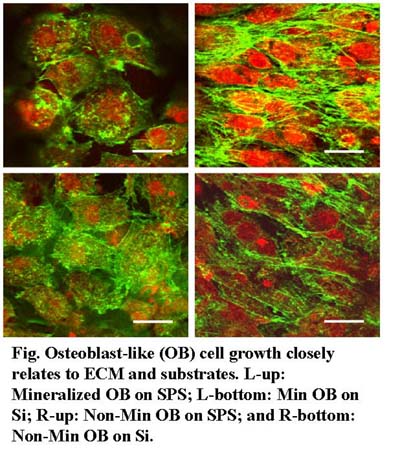

Bone tissue engineering plays an important role in regeneration and tissue repair. Understanding how biomineralization occurs in the extracellular matrix (ECM) of bone cells is crucial to the understanding of bone formation and the development of a successfully engineered bone tissue scaffold.
It is still unclear how the ECM mechanical properties affect the protein-mineral interaction in early stages.
We investigated the longitudinal mineralization properties of MC3T3-E1 cells and their ECM using shear modulation force microscopy, X-ray diffraction (XRD), scanning electron microscopy, electron dispersive X-ray spectroscopy, and confocal laser scanning microscopy (CLSM).
On substrates conducive to ECM network production the elastic modulus of mineralizing cells increased at time points corresponding to mineral production, whereas that of the non-mineralizing cells did not vary over time. The elastic modulus of the ECM fibrils underwent significant changes for the mineralizing cells, which were not observed in the non-mineralizing cells. The presence of hydroxyapatite in mineralizing cells and the absence thereof in the non-mineralizing ones were confirmed by XRD. CLSM showed that a restructuring of actin occurred only for mineral-producing cells. These results show that the correct and complete development of the ECM network is required for osteoblasts to mineralize. This in turn requires a suitably prepared synthetic substrate for bone development to succeed in vitro.
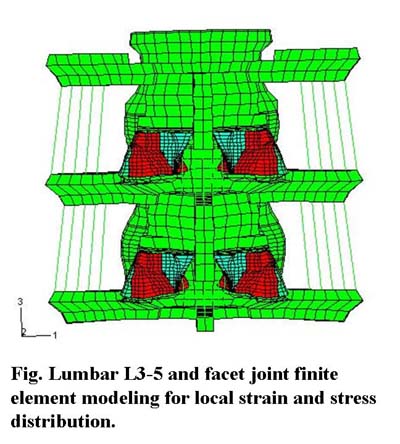

Eighty percent of Americans will experience low back pain (LBP) in their lifetime; for the majority of LBP patients, the etiology continues to be poorly understood.
A known cause of LBP is the activation of mechano-nociceptors innervating lumbar facet joint capsules. In isolated, but intact, nerve - capsule (as well as nerve - skin & nerve - muscle) mechanoreceptors and no-ciceptors appear to encode the local stress rather than the local strain during loading.
In-vivo, it is virtually impossible to directly measure capsule stress; however, robust estimates of capsule stress are possible using the finite element method (FEM). Previous FEM models of the lumbar spine have not included accurate representations (geometry or material properties) of the facet joint (FJ) and its posterior capsule. The purpose of this study was to create a geometrically correct FEM model of the lumbar FJ, incorporate it into an existing lumbar spine model, and subject it to normal physiological loading conditions.

Osteoporosis is a disease characterized by decreased bone mass and progressive erosion of the microstructure. As a result, bone is at higher risk for developing chronic and traumatic fractures at key skeletal sites. Therapeutic ultrasound may offer a potential non-pharmacologic, site-specific intervention for treatment of osteoporotic bone loss.
It has been proposed that LIPUS acts as an alternating pressure wave, creating local pressure gradients within bone's micro porosities, which may result in the production of anabolic shear forces on cell membranes, changes in local solute concentrations or initiation of local fluid flow exchange and interaction. In vitro studies have shown that LIPUS enhances osteoblast prolifera-tion and endochondral bone formation and in vivo studies have shown LIPUS to be anabolic in fresh fractures, enhancing endochondral bone formation, mineral density and mechanical strength. However, the efficacy of therapeutic ultrasound as a treatment for intact bone remains unclear. The goal of this study was to explore the therapeutic potential of LIPUS for treatment of bone loss associated with estrogen deficient osteopenia using high resolution ?CT imaging and finite element models. We hypothesized that ultrasound induced microstreaming can inhibit bone loss and maintain mechanical strength, and that ultrasound pulse duration plays a role in bone's response by modulating bone loss and maintaining mechanical strength.
These findings support the hypothesis that LIPUS can inhibit bone loss and preserve bone strength under conditions of estrogen deficient osteopenia. The results indicate that therapeutic ultrasound acts to decrease bone loss by minimizing trabecular thinning and maintaining bone's plate-like structure. Subsequently, bone is capable of withstanding larger loads and is less likely to develop stress concentrations that may result in localized bone failure.
These findings support the hypothesis that LIPUS can inhibit bone loss and preserve bone strength under conditions of estrogen deficient osteopenia. The results indicate that therapeutic ultrasound acts to decrease bone loss by minimizing trabecular thinning and maintaining bone's plate-like structure. Subsequently, bone is capable of withstanding larger loads and is less likely to develop stress concentrations that may result in localized bone failure.
Orthopedic Bioengineering Research Laboratory
Department of Biomedical Engineering
State University of New York Stony Brook
Bioengineering Building, Rm 215
Stony Brook, NY 11794-5281
p: 631.632.4382 f: 631.632.8577 e: yi-xian.qin@sunysb.edu

