The SynCardia total artificial heart (TAH) is the only Food and Drug Administration (FDA) approved TAH for both bridge-to-transplant and as destination therapy under Humanitarian Use Device (HUD) for patients with congestive heart failure. It is a pulsatile bi-ventricular mechanical circulatory support device that replaces the heart in patients with end-stage congestive heart failure. It pumps blood via pneumatically driven diaphragms altering the volume of the ventricular chambers. Flow in and out of the ventricles is controlled by mechanical heart valves. While over 1,300 patients have been successfully implanted with the TAH, its current size (70 cc) precludes implanting it in smaller patients and pediatric use. To address this unmet clinical need, the aim of this study is to evaluate the viability of scaled down versions of the device, by studying flow patterns in TAHs of differing sizes and quantifying their thrombogenic potential.
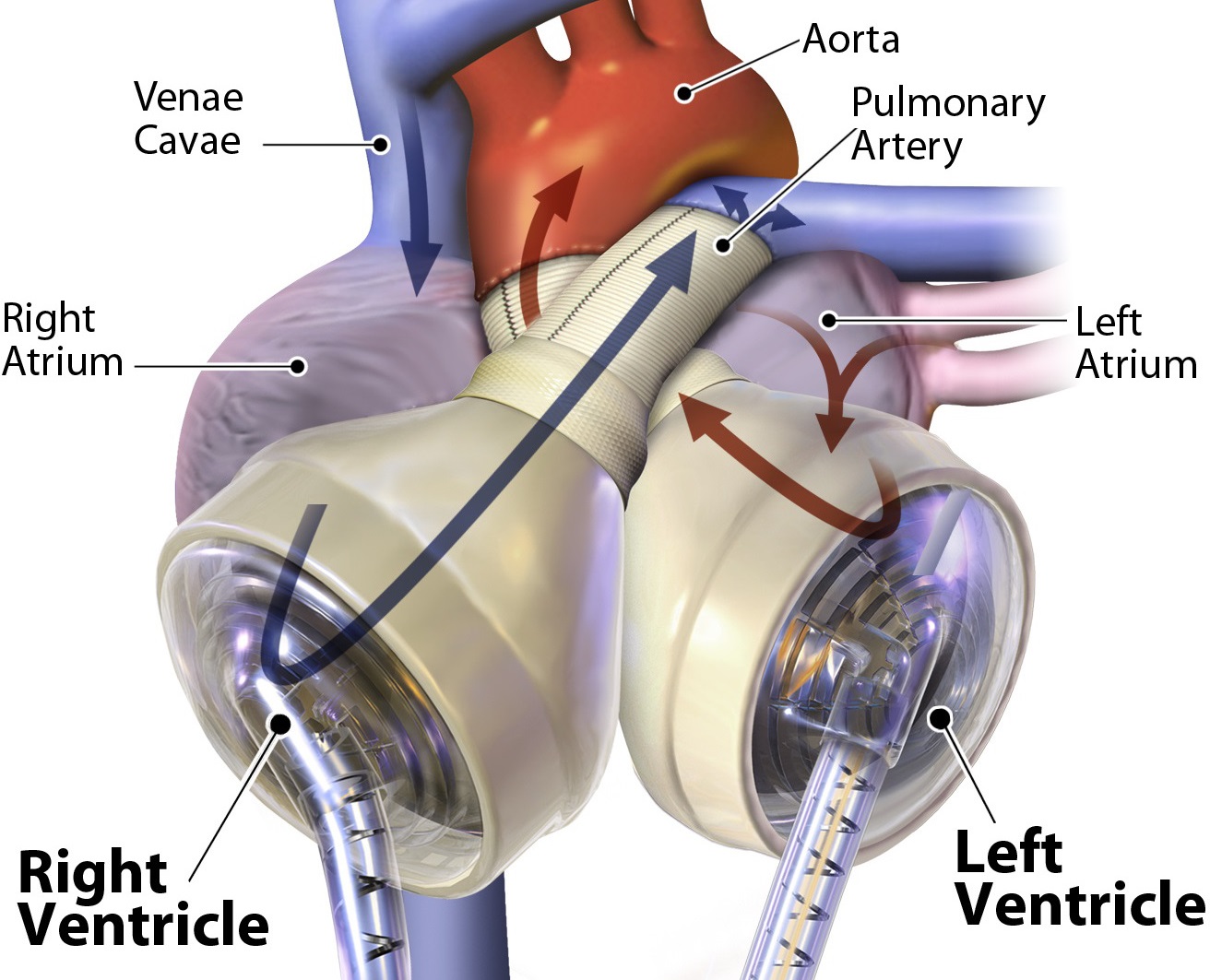
Simulations of the blood flow through TAH were conducted with fluid-structure interaction model of the full cardiac cycle where the valve leaflets were free to move and the motion of the deforming diaphragms was predefined. The blood was modeled as a multiphase fluid with suspended platelet particles. Platelet trajectories were calculated and flow stress accumulations computed along their corresponding trajectories. The thrombogenic potential was quantified for a large population of platelets flowing through the devices. The models were solved in ANSYS Fluent using in-house user defined functions (UDFs) for the valves-fluid coupling and the diaphragm motion. The simulations successfully captured complex flow patterns during various phases of the TAH cardiac cycle, including the opening and closing of the valves and regurgitation through them while closed.
Publications
External links
- How to mend a broken heart, Alex O’Brien, Mosaic Science, June 2, 2015
- A Change of Heart, New York Times, March 21, 2016