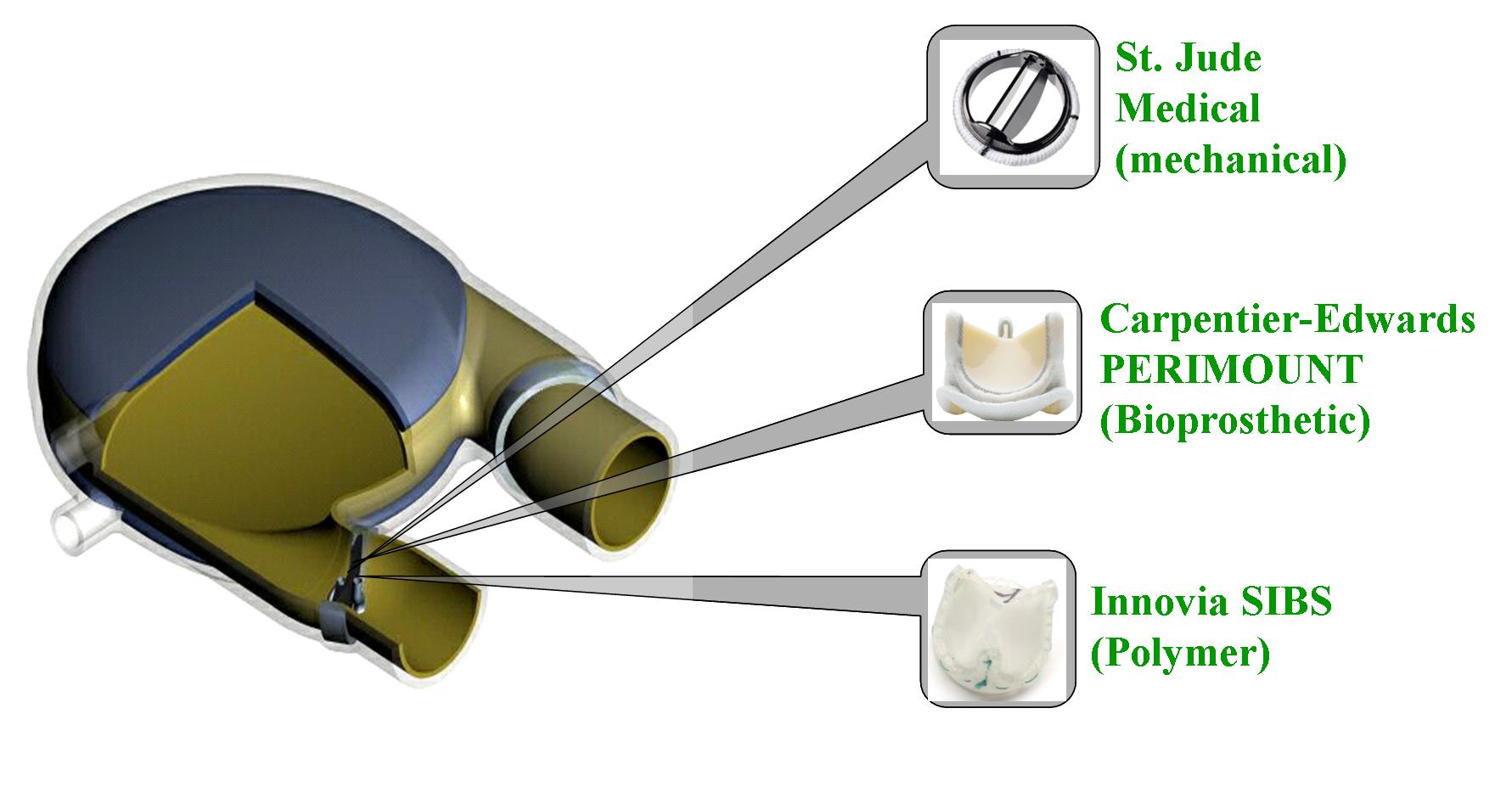
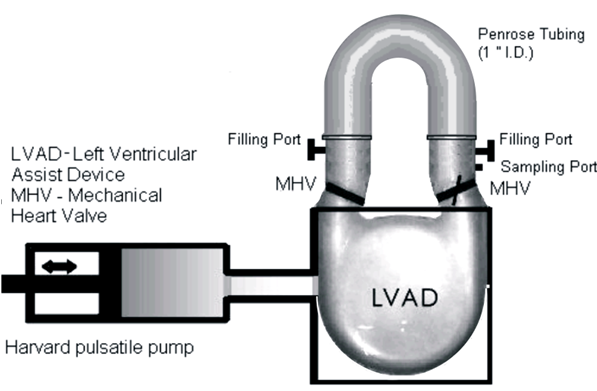
Patients with severe valvular diseases require heart valve replacement as a necessary treatment. Approximately 2 million individuals have received prosthetic heart valves (PHVs), with 120,000 valves implanted annually in the United States. About 60% of PHVs are categorized as mechanical heart valves (MHVs), with the remainder described as bioprosthetic or polymer valves. Each valve type has its advantages and disadvantages, with primary focus being placed on the risk of thromboembolism due to platelet activation induced by non-physiologic flow patterns, typically blamed on valve leaflet design. Polymer valves have been touted as a solution to this issue, owing to the design of leaflets that mimic geometry found in natural valves, and newer products utilize materials that show high biocompatibility and reduced long-term degradation. Our objective is to quantify the thrombogenic potential of 3 types of PHVs via platelet activation, and to identify which valve is least likely to cause long-term thromboembolic complications. The three valves utilized in our study are the St. Jude Medical bileaflet MHV, a Carpentier-Edwards bioprosthetic valve, and a trileaflet polymer valve, provided by Dr. Richard Schoephoerster. The valves are mounted left ventricular assist device (LVAD), kindly provided by Dr. Klaus Affeld, filled with a platelet mixture at physiological concentration, and connected to a Harvard Apparatus pulsatile blood pump. We conduct 30-minute experiments, with platelets sampled at regular intervals for the platelet activation state (PAS) assay, which allows quantification of platelet activation, and therefore thrombogenic potential of each valve. Previous studies examined platelet activation in 3 PHVs of prior designs, as well as the thrombogenic potential of bileaflet and monoleaflet MHVs.