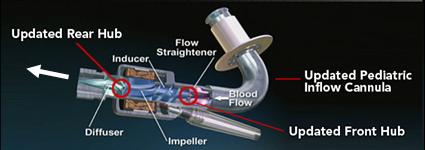
Left ventricular assist devices (LVADs) have become the standard alternative to cardiac transplant for patients with advanced-stage heart failure due to the inadequate availability of donor hearts. To assist failing ventricular function, blood is mechanically pumped out from the apex of the ventricle by the LVAD and rejoins the circulation at the aorta. Pneumatic and rotary LVADs have been developed, and the latter has gradually become more prominent due to its high durability and compact design.
LVADs generate a non-physiological and dynamic high shear environment, and blood is exposed to the risk of shear-induced damage while passing through, leading to post-implant thrombotic and thromboembolic complications. Thus, anticoagulant and antiplatelet therapy are mandated for device recipients. However, management of such therapy is challenging, and may lead to secondary complications (e.g., hemorrhage).
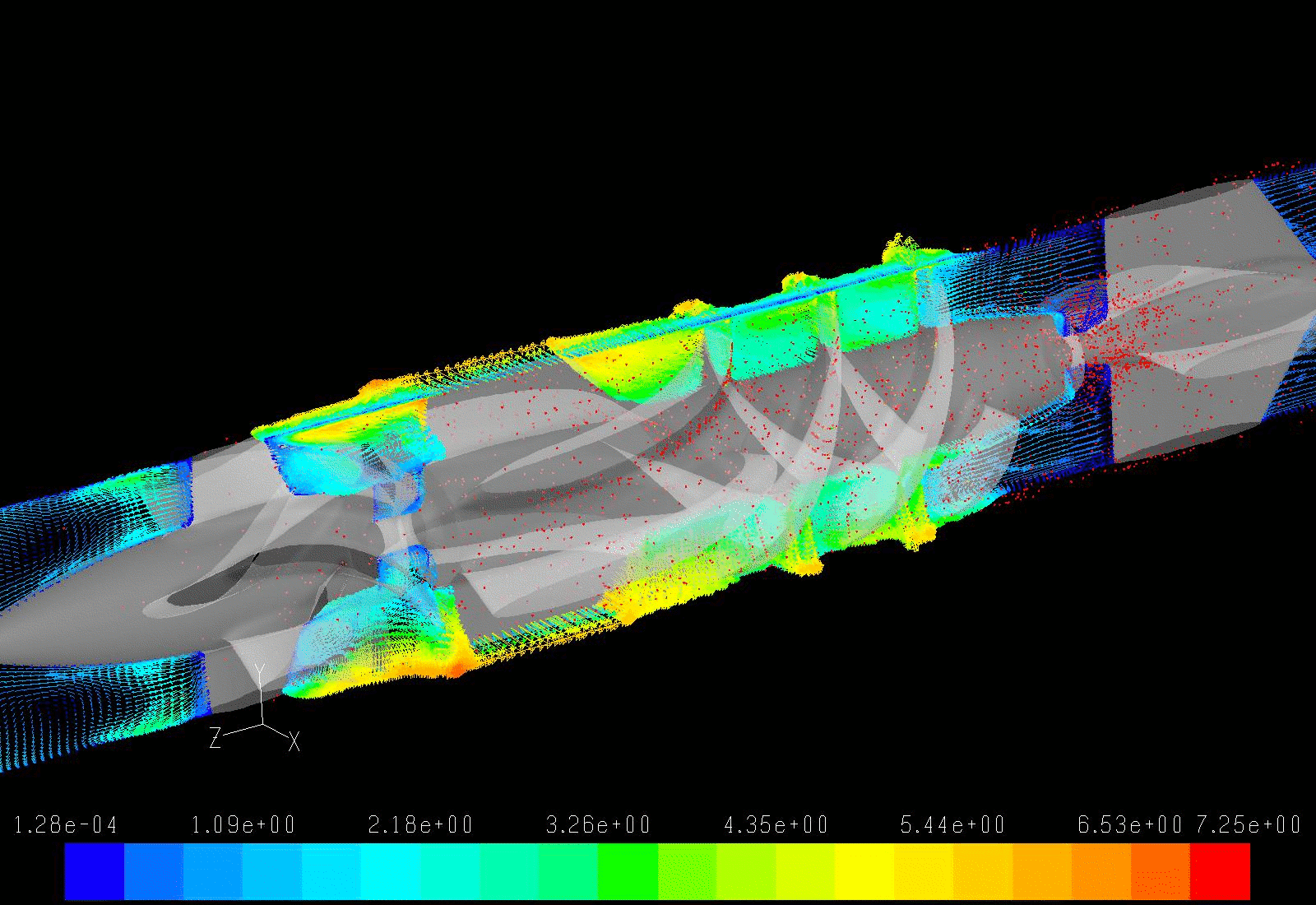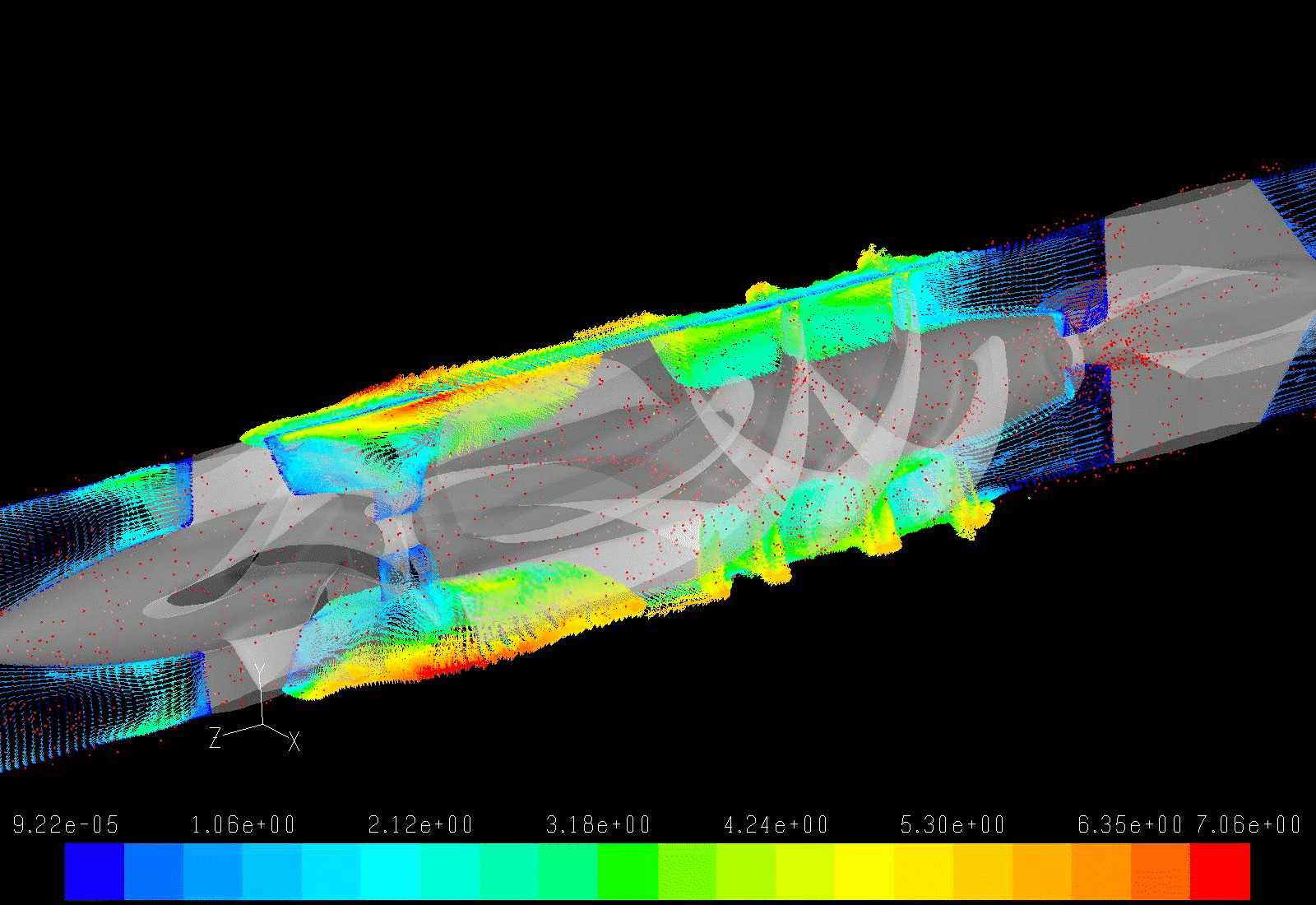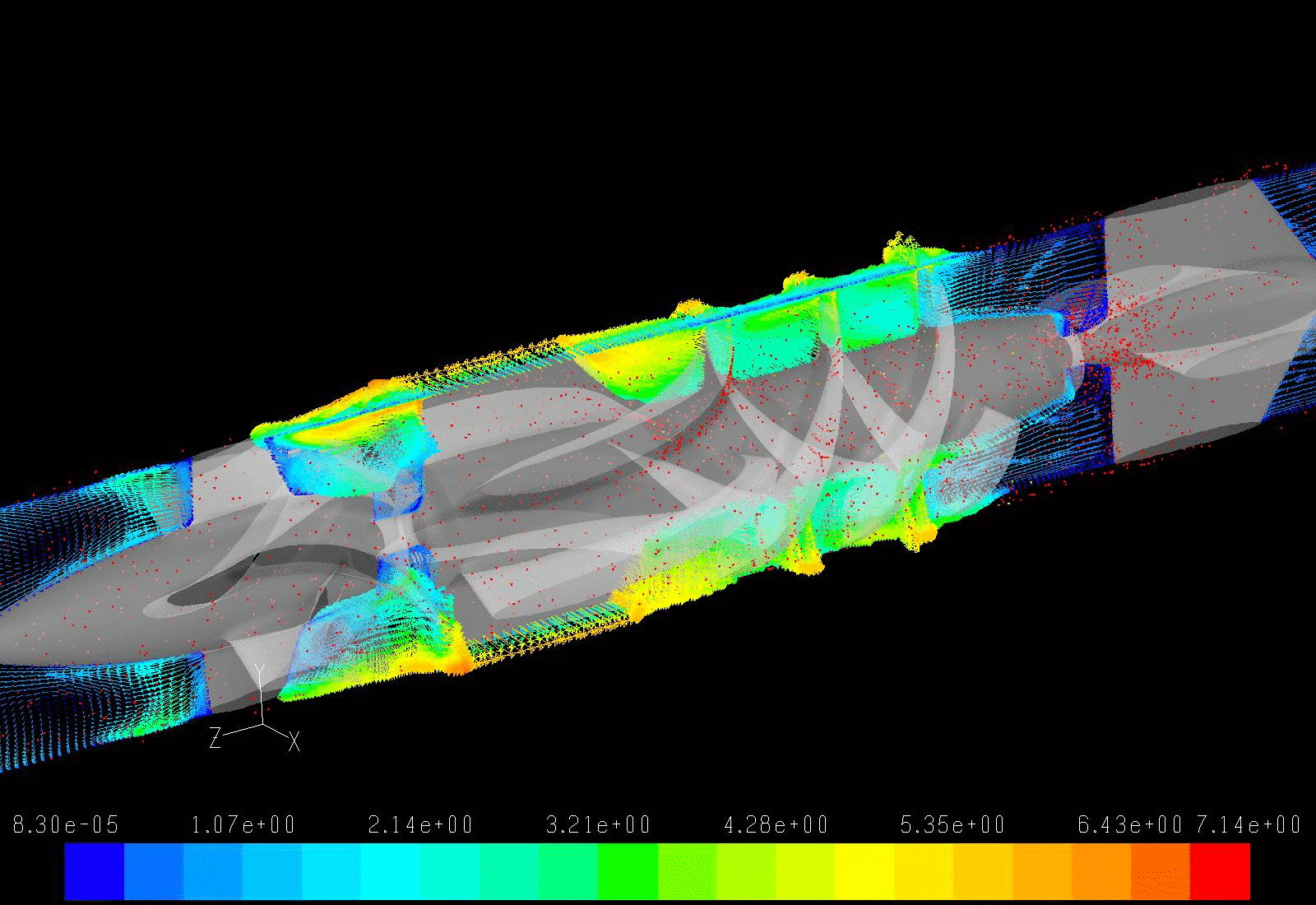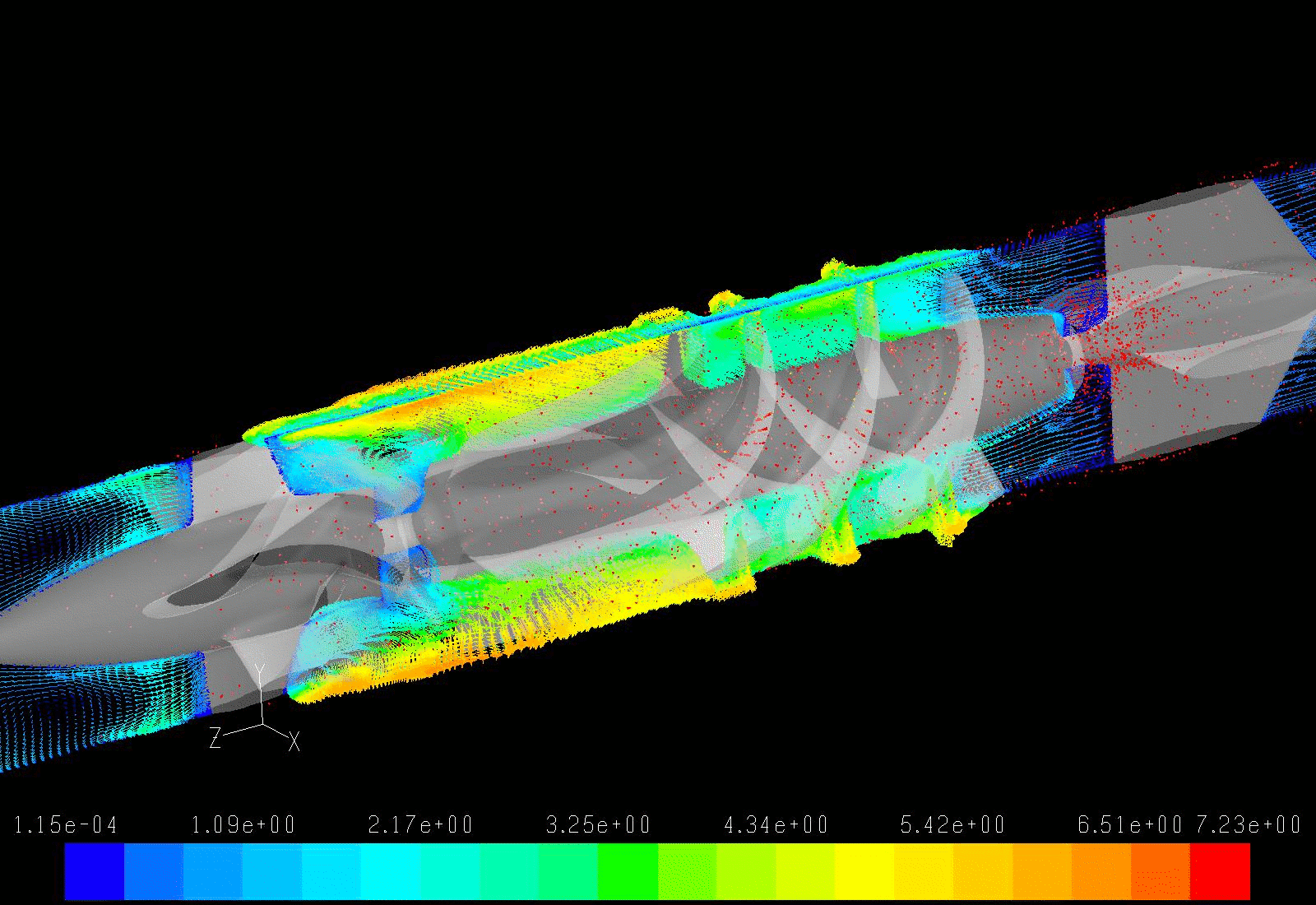
Pump Design Optimization
Our lab aims to optimize the LVAD design to minimize the highly dynamic shear environment and achieve the ultimate goal of reducing or eliminating device-related shear-induced blood damage. We collaborate with industry partners to evaluate and modify LVAD designs through numerical simulations to predict device thrombogenicity potential. This is validated with benchtop experiments and animal studies utilizing industry-provided prototypes.
Evaluation of Antiplatelet Efficacy in LVADs
The role of the high shear LVAD environments on the efficacy of antiplatelet drugs is poorly understood. Our group has conducted experiments analyzing platelets treated directly with aspirin or dipyridamole, or platelets taken from blood donors who ingested aspirin, after flow through an in vitro LVAD loop. Results show a mild reduction in platelet activation after aspirin or dipyridamole treatment, and more significant reduction with design modification of the LVAD.
Implantation Configuration Optimization
In addition to pump design optimization, we numerically simulate the deleterious effect of non-optimized implantation configuration on the overall device thrombogenic potential. Several components, including the inflow and outflow cannulae, are required for connecting an LVAD to the recipient’s circulation system. These components are connected to the pump prior to the implantation, and the surgeon is tasked with finding the best anatomical fit, with limited guidance provided by the manufacturer, leading to various surgical and post-surgical complications. Our group aims to optimize the implant configuration in silico.